Formulative (Industrial) Pharmacy – I Past Years RGUHS Download Question Paper PDF BPharmacy 5th…
Regulatory affairs NOTES Download Notes PDF BPharmacy 7th Semester 2022📑 BP702T Industrial Phar…
Regulatory Affairs Industrial Pharmacy Handwritten IP-2 U-3 Download Notes PDF BPharmacy 7th Sem…
 B.Pharmacy Education
B.Pharmacy Education
Download PDF NotesBPharmacy 5th Semester PCI Download PDFUnit 5 Cosmetics Handwritten Notes Form…
 B.Pharmacy Education
B.Pharmacy Education
Download PDF NotesBPharmacy 5th Semester PCI Download PDFUnit-1 Formulative (Industrial) Pharmac…
 B.Pharmacy Education
B.Pharmacy Education
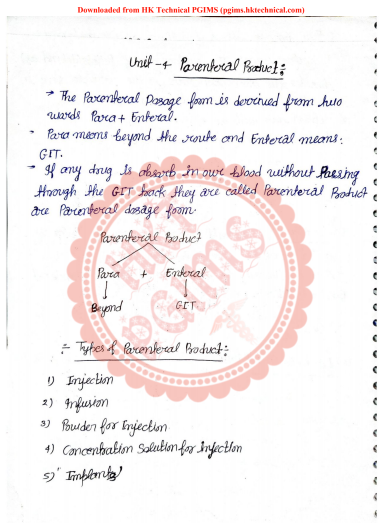
Download PDF NotesBPharmacy 5th Semester PCI Download PDFUnit-4 Parenteral Product Handwritten N…
 B.Pharm 7th Semester Notes
B.Pharm 7th Semester Notes
Biostatistics in Pharmaceutical Product Development | Industrial PharmacyWhat is Biostatistics?S…
BP702T Industrial Pharmacy-II NotesB Pharmacy 7th SemesterContent:
UNIT-I
Pilot plant scale up tec…
Spiking:Spiking can be defined as the addition of unknown amount of a compound to a standard sam…
Quality Risk Management (QRM):Quality Risk Management is a systematic process for the assessment…
Process Validation:Process Validation is defined as the collection and evaluation of data, from …
Performance Qualification (PQ):Document verification that the equipment or system operates consi…
Operational Qualification (OQ):Document verification that the system or sub-system performs as i…
Installation Qualification (IQ):The performance of tests to ensure that the installations used i…
GAP Analysis:Identification of critical element of a process which are available at the SU but a…
Sending Unit (SU):The involved discipline at an organisation from where a designated product, pr…
Finished Pharmaceutical Product (FPP):FPP or Finished Pharmaceutical Product can be defined as a…
Drug Master File (DMF):DMF or Drug Master File is teh detailed information regarding a specific …
Design Qualification (DQ):Design qualification is the document evidence that the premises, suppo…
 B.Pharm 7th Semester Notes
B.Pharm 7th Semester Notes
CDSCO Industrial PharmacyNotes PDF DownloadB.Pharmacy 7th Semester
Enter Your Institute & Institute Address to get more benefits